We
took a unit quiz today. I feel like I did well and knew the material that I was
being tested over, but I guess I will not know for sure until the grades are in
which will hopefully be soon. I only had semi-major issues with one question,
so I skipped it and came back to it. When looking at it for a while the second
time around, I noticed something I had not before and easily came to the
correct answer. I feel like I was successful with both the questions from this
unit and the review questions from last unit.
Wednesday, September 23, 2015
Beanium Lab
Yesterday,
we did an activity to further our understanding on isotopes. Isotopes are atoms
that belong to the same element, but the number of neutrons varies
causing the atomic mass to vary between the different isotopes. This was
demonstrated using different colored beans to represent the isotopes of the
fictional element “beanium”. We sorted all of the beans by color and found the
average mass of each individual bean for that specific color. Not surprisingly,
each color had a different average mass, much like the isotopes of an element.
We used these numbers and percent of abundance to find the average atomic mass
of beanium. The picture shows all of our beans sorted by color.
Sunday, September 20, 2015
Atomic Theory Notes
In class, we learned about Dalton's Atomic Theory and JJ Thomson's, Rutherford's, and the current atomic model. Following are a summary of the notes for each of these model's:
- Dalton's Atomic Theory
- Elements are made of tiny particles called atoms.
- All atoms of a given element are identical.
- The atoms of a given element are different from those of any other element.
- Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers and types of atoms.
- Atoms are indivisible in chemical processes. That is, atoms are not created or destroyed in chemical reactions. A chemical reaction simply changes the way the atoms are grouped together.
- JJ Thomson
- Used a cathode ray tube to show that atoms of any element could be made to give off what we now know as electrons
- He concluded that every atom has these tiny, negative charged particles
- He also knew that atoms have a neutral charge, so there must be an equal amount of positively charged particles
- Also known as "plum pudding model" or "chocolate chip cookie model"
- No nucleus
- Rutherford
- Shot high velocity alpha particles at an atom expecting there to be very little to deflect the particles
- Some particles bounced back
- Concluded that the only way they could have bounced back was if most of the atom's mass was concentrated in a nucleus
- Eventually discovered that the nucleus contained protons
- Current model
- Also known as the "cloud model"
- Electrons move around nucleus in a cloud
- Electron location is pinpointed using proabability
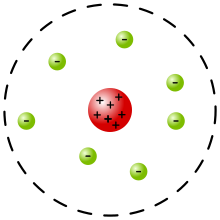
Unit 2 Pretest
While taking the pretest for this unit, I saw some things that I have vaguely remembered from past units in school, but that information was still fairly blurry in my memory. The rest of the information that was on the pretest I have never seen before. I look forward to learning a lot of new things this unit while reinstated the things I have already learned.
Thursday, September 17, 2015
Atomic Inference Activity
Yesterday we did an inferencing activity that tied in with our lesson of atomic models. In our activity we picked up a covered plate that contained a marble. In the plate, there was a pattern of raised lines that the marble could not pass over. By rolling the marble around and listening to where it was rolling, we had to try and guess what the design inside was. This ties in with our lesson in that one model we learned about, the Rutherford model, was created through inferences. He shot high velocity alpha particles at an atom expecting that there would be very little to deflect the particles. Most particles acted like he expected, but some bounced back. He concluded that the only way the particles could bounce back is that if most of the mass in an atom is concentrated in a nucleus. In both our activity and Rutherford's experiment, we had to use observations of behavior to determine what was located inside something.
Monday, September 14, 2015
Nomenclature Post #2
I can apply the information I learned during the Frontier Chemistry project to the real world in that I may find myself with an ailment in the woods and have to use my knowledge of medicinal plants in order to treat myself. Much like our essay over the unit, my life my depend on my ability to properly identify plants and know their medicinal uses. These plants are actually used to treat maladies, it is not just some abstract concept only relevant to this project.
Nomenclature Post #1
What I found most difficult about this task was having to find the plants out in the wild. I found this difficult because I was not really sure what I was looking for at the time, so I felt like I was just taking pictures of random plants and hoping for the best. Also, when it came time to identifying the plants from the pictures that I took, sometimes it was difficult to see the small details that could help identify the plant. Sometimes the way the light hit the plant made it look like a slightly different color than the picture I was trying to match it with, making me concerned that I was looking at 2 different plants.
Subscribe to:
Comments (Atom)
