- Dalton's Atomic Theory
- Elements are made of tiny particles called atoms.
- All atoms of a given element are identical.
- The atoms of a given element are different from those of any other element.
- Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers and types of atoms.
- Atoms are indivisible in chemical processes. That is, atoms are not created or destroyed in chemical reactions. A chemical reaction simply changes the way the atoms are grouped together.
- JJ Thomson
- Used a cathode ray tube to show that atoms of any element could be made to give off what we now know as electrons
- He concluded that every atom has these tiny, negative charged particles
- He also knew that atoms have a neutral charge, so there must be an equal amount of positively charged particles
- Also known as "plum pudding model" or "chocolate chip cookie model"
- No nucleus
- Rutherford
- Shot high velocity alpha particles at an atom expecting there to be very little to deflect the particles
- Some particles bounced back
- Concluded that the only way they could have bounced back was if most of the atom's mass was concentrated in a nucleus
- Eventually discovered that the nucleus contained protons
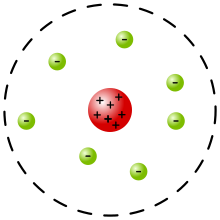
- Current model
- Also known as the "cloud model"
- Electrons move around nucleus in a cloud
- Electron location is pinpointed using proabability
I love the thoroughness of your notes. It really seems like you have a firm grasp on the information. Thank you for sharing the pictures with your notes, it really helps aid them and adds color to the post!
ReplyDelete